UNITY Complete - Where Others Stop, We Keep Going.
UNITY directly measures disease-causing mutations in the pregnancy to deliver an accurate fetal risk assessment as a first-line screening for the general obstetric population.
Detect more affected pregnancies
UNITY Fetal Risk Screen leverages cell-free DNA to provide direct insights to the fetus, translating to ~3X increase in detection of affected pregnancies compared to traditional carrier screening.1
The only cell-free genetic screening test that can provide a fetal risk for recessive conditions.
UNITY Fetal Risk Screen will first determine if the mother is a carrier for a recessive condition included in the test. If she is determined to be a carrier, single-gene NIPT (sgNIPT) will be performed from the same sample to determine the risk that the fetus is affected for the condition of interest. This is done via cell-free-DNA (cfDNA) and proprietary QCTTM technology. UNITY Fetal Risk Screen is the only screening test that can clarify risk down to 1 in 5,000 or up to 9 in 10 for maternal carriers.
Reassure 99% of pregnant patients, early in their pregnancy
The majority of patients will learn they are at a significantly reduced risk of an affected fetus.
Less than 1% of patients will receive a high fetal risk report, as early as the first trimester.1
One blood draw. One patient bill. Fetal risk for both chromosomal and recessive conditions recommended by ACOG*.
All conditions on our panels are aligned to ACOG recommended conditions REF, with the option to add-on fetal antigens for clinically appropriate patients. No matter which test combination is right for your patient, clear reporting and direct insights to fetal risk make it easy to interpret results.
Designed for a general obstetric population
Screens for conditions aligned to ACOG recommendations for a general OB population.2,3
Cell-free DNA analysis does not require a partner sample.
UNITY complete requires one blood draw from mom, as early as 10 weeks.
UNITY Complete leverages circulating cell-free DNA to provide direct fetal insights for both aneuploidies and recessive conditions. This equates to not only more informative insights, it also overcomes workflow complexities associated traditional carrier screening.
Relying on a male partner’s sample, as is the case with traditional carrier screening has its challenges in clinical practice as it is impacted by factors such as misattributed paternity (10%) or lack of partner follow up (58%).
UNITY Complete.
UNITY Complete offers genetic insights for both recessive and chromosomal conditions aligned to genetic screening recommendations by ACOG.2,3 Patients who are alloimmunized or are RhD-negative may also benefit from UNITY Fetal Antigen™ tests which can be added to UNITY Aneuploidy anytime during pregnancy.

UNITY Fetal RiskTM Screen
- Cystic Fibrosis
- Sickle Cell Disease
- Beta-Thalassemia
- Spinal Muscular Atrophy
- Alpha-Thalassemia
- 5-Gene Panel
- Canavan Disease
- MCAD Deficiency
- Tay-Sachs Disease
- Familial Dysautonomia
- Smith-Lemli-Opitz Syndrome
- PMM2-Congenital Disorder of Glycosylation
- DMD-Associated Dystrophinopathies*
- Phenylalanine Hydroxylase Deficiency (PKU)
- Fragile X Syndrome*† (optional)

UNITY AneuploidyTM Screen
- Trisomy 21**
- Trisomy 18**
- Trisomy 13**
- Sex Chromosome Aneuploidies:
X, XXY, XYY, XXX - Zygosity (included for twins)
- 22q11.2 Microdeletion Syndrome (optional)**
- Fetal Sex (optional)**

UNITY Fetal AntigenTM Tests
UNITY RBC Fetal Antigen NIPT
- Big C, little c, D, E, Fya (Duffy), jka (Kidd), jkb (Kidd), K (Kell), k, M, N, Big S, little s, U
- *M and N antigens must be selected at ordering and cannot be added after the test is submitted
UNITY Platelet Fetal Antigen NIPT
- HPA-1a (with HLA-DRB3*01:01 when applicable), HPA-1b, HPA-2a, HPA-2b, HPA-3a, HPA-3b, HPA-4a, HPA-4b, HPA-5a, HPA-5b, HPA-9a, HPA-9b, HPA-15a, HPA-15b
Access a Sample Report.
Advanced Fetal Risk Assessment withDual Panel Flexibility.
UNITY Fetal Risk Screen is the first-and-only non-invasive prenatal test that leverages Quantitative Counting Template™ (QCT™) technology to deliver a precise fetal risk for up to 14 prevalent and actionable recessive and X-linked conditions — all from a single maternal blood draw as early as 9 weeks into pregnancy. No partner sample required.
The Latest Data With UNITY Complete.
Additional Provider Resources.

Product Information

MFM Perspective: UNITY Fetal Antigen NIPT

Test Requisition Form
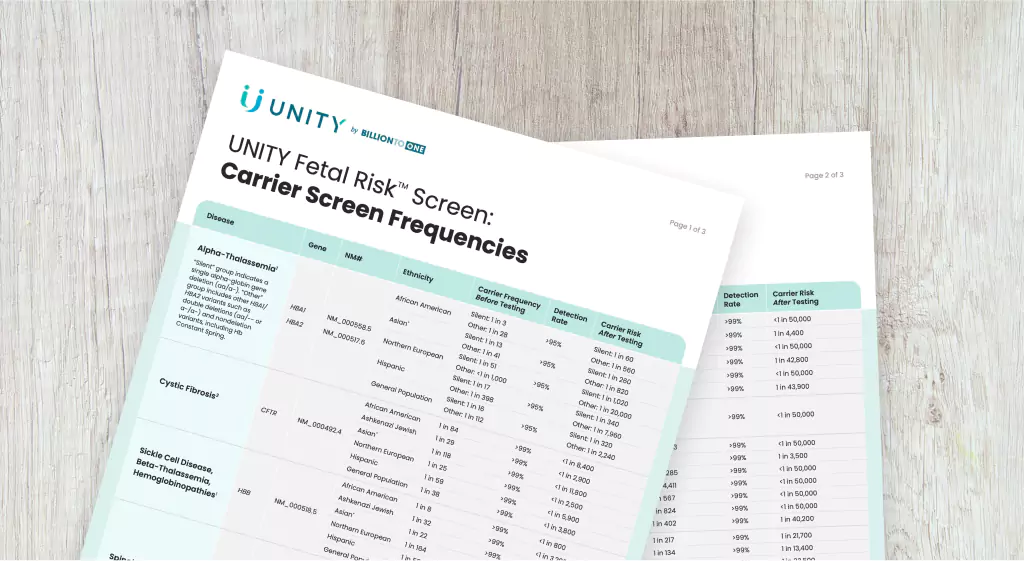
Carrier Frequencies Table
Frequently Asked Questions
UNITY Complete takes advantage of a pregnancy's DNA (circulating cell-free DNA, ccfDNA) floating in the maternal bloodstream to assess for genetic changes. Some of these genetic changes are extremely small - like changes to single genes that can cause recessive conditions. Some of these genetic changes involve the presence of an entire extra chromosome (aneuploidies).
Our specialized QCT technology enables us to be able to count the genetic information we see from both mom and baby in a blood sample, and determine if there are differences that could indicate a baby has a higher chance of being affected with one of these conditions.
UNITY Complete offers comprehensive genetic screening from a single maternal blood sample - no partner sample required. The specific insights you receive depend on the tests you order based on your patient’s clinical needs.
UNITY Aneuploidy™ Screen checks for chromosomal conditions caused by extra or missing chromosomes, including Trisomy 21, Trisomy 18, Trisomy 13, Monosomy X, XXX, XXY, and XYY. This test also provides fetal sex determination and, for twin pregnancies, identifies whether the twins are identical or fraternal.
Depending on your patient's clinical history, you may also order:
• UNITY Fetal RhD NIPT for patients with RhD-negative blood type.
• UNITY Fetal Antigen NIPT for patients who are alloimmunized to certain antigens.
• 22q11.2 microdeletion analysis
UNITY Fetal Risk™ Screen offers two panels to assess carrier status for up to 14 recessive and x-linked conditions:
• ACOG Guideline Panel (5 conditions): Screens for conditions recommended by the American College of Obstetricians and Gynecologists (ACOG), such as cystic fibrosis, spinal muscular atrophy, sickle cell disease, alpha-thalassemia, and beta-thalassemia.
• ACOG Guideline PLUS Panel (14 conditions): Includes all conditions from the ACOG Guideline Panel, plus additional conditions prevalent in Ashkenazi Jewish and pan-ethnic populations such as Tay Sachs Disease, DMD-Associated Dystrophinopathies, Phenylalanine Hydroxylase Deficiency (PKU), and others.
If a patient is found to be a carrier for any of these conditions, fetal testing will automatically be performed using cell-free DNA to assess the likelihood of the fetus being affected. The fetal risk result will indicate whether the risk is low or high for each condition. Please note that fetal risk assessment may not be available for certain cases, such as twin pregnancies or those involving egg donation.
Additionally, you may choose to order carrier screening for Fragile X syndrome. If the patient is a carrier, our assay can perform cell-free DNA analysis to determine the fetus's sex, as male fetuses are at a higher risk of developing Fragile X syndrome.
We believe all pregnant patients should have access to UNITY Complete.
We accept ALL insurances, including Medicaid, and are in-network with the majority of insurance plans across the U.S. We understand that each patient's insurance and financial situation is unique. Our dedicated patient services team is available to assist with payment plans or financial assistance for eligible patients. If you have any questions about costs or how to support your patients, please email us at support@unityscreen.com or call 650-460-2551.
UNITY Complete is ordered by your healthcare provider. If you decide you want UNITY Complete, please call us at 650-460-2551 or email us at support@unityscreen.com and we can get your provider the information they need to get started.
UNITY Aneuploidy Screen results are typically reported as either low-risk or high-risk.
Low-Risk Fetus: This indicates a very low chance that the pregnancy is affected by the conditions tested, though it does not completely eliminate the possibility.
High-Risk Fetus: This indicates an increased likelihood that the pregnancy may be affected by a specific condition. In the case of a high-risk result, follow-up testing such as chorionic villus sampling (CVS), amniocentesis, or post-birth evaluations is generally recommended.
Important: A high-risk result does not guarantee an unhealthy pregnancy and does not rule out other genetic conditions or birth defects.
UNITY Fetal Risk Screen evaluates maternal carrier status for various conditions. If the patient is identified as a carrier, fetal risk assessment will be automatically performed using cell-free DNA. The results will provide either a low-risk or high-risk determination for each condition tested.
Get Started.
Ready to implement a new standard in prenatal care in your practice? Order a test kit to get started.
References
1. Wynn J, et al. Performance of single-gene noninvasive prenatal testing for autosomal recessive conditions in a general population setting. Prenat Diagn. 2023 Sep; 43(10):1344-1354. doi: 10.1002/pd.6427. Epub 2023 Sep 6. PMID: 37674263
2. Carrier screening for genetic conditions. Committee Opinion No. 691.American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;129:e41–55.
3. Practice Bulletin No. 163: Screening for Fetal Aneuploidy. Obstet Gynecol. 2016 May;127(5):e123-e137. doi: 10.1097/AOG.0000000000001406. PMID: 26938574.
4. Deignan, Joshua L., et al. Updated recommendations for CFTR carrier screening: A position statement of the American College of Medical Genetics and Genomics (ACMG). Genetics in Medicine 25.8 (2023): 100867.






